Time Is of the Essence
Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Airway, ventilatory and circulatory support, oxygen administration, and management of seizures should not be delayed to administer CYANOKIT.1
The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.1
Signs and symptoms of acute systemic cyanide poisoning may develop rapidly, depending on the route and extent of cyanide exposure. If cyanide poisoning is known or suspected, treat with CYANOKIT—an FDA-approved treatment option for cyanide poisoning.1Key Facts About CYANOKIT:
- CYANOKIT for intravenous infusion is indicated for the treatment of known or suspected cyanide poisoning. If clinical suspicion of cyanide poisoning is high, CYANOKIT should be administered without delay1
- CYANOKIT can be used to treat multiple sources of cyanide poisoning, including smoke inhalation and ingestion1
- Hydroxocobalamin, the active ingredient in CYANOKIT, has been used in France since the 1980s, was formally licensed there in 1996, and was approved in the United States in 2006 to treat known or suspected cyanide poisoning6,7
Clinical Experience
Randomized clinical trials in humans are not possible due to ethical considerations, therefore evidence in controlled trials of the effectiveness of hydroxocobalamin for the treatment of cyanide poisoning was obtained primarily from studies in animals. However, 4 open-label, uncontrolled clinical studies were conducted in known or suspected cyanide poisoning victims.1Experience in Known or Suspected Cyanide Poisoning Victims
A total of 245 patients received hydroxocobalamin treatment in these studies. Systematic collection of adverse events was not done in all of these studies and interpretation of causality is limited due to the lack of a control group and due to circumstances of administration (e.g., use in fire victims). Adverse reactions reported in these studies listed by system organ class included1:- Cardiac disorders: ventricular extrasystoles
- Investigations: electrocardiogram repolarization abnormality, heart rate increased
- Respiratory, thoracic, and mediastinal disorders: pleural effusion
Smoke-Inhalation Studies
A prospective, uncontrolled, open-label study spanning almost 7 years was carried out in 69 subjects who had been exposed to smoke inhalation from fires. Subjects had to be over 15 years of age, present with soot in the mouth and expectoration (to indicate smoke exposure), and have altered neurological status. The presence of cyanide poisoning, defined by a pretreatment blood cyanide concentration ≥39 µmol/L was confirmed in 42 of the 63 patients with evaluable data (67%). The median hydroxocobalamin dose was 5 g with a range from 4 g to 15 g.1,6Following the administration of hydroxocobalamin, 50 of 69 subjects with cyanide poisoning (73%) survived.1,6
The most common adverse events for this study were chromaturia (n=6), pink or red skin discoloration (n=4), hypertension (n=3), erythema (n=2), and increased blood pressure (n=2).6
Two additional retrospective, uncontrolled studies were carried out in subjects who had been exposed to cyanide from fire or smoke inhalation. Subjects were treated with up to 15 g of hydroxocobalamin. Survival in these two studies was 34 of 61 (56%) for one study, and 30 of 72 (42%) for the second.1
Ingestion or Inhalation Study
A retrospective chart review over a period of 15 years was compiled by identifying 14 patients who had been exposed to cyanide from sources other than from fire or smoke (i.e., ingestion or inhalation). Subjects were treated with 5 g to 20 g of hydroxocobalamin. Eleven of 12 subjects whose blood cyanide concentration was known had initial blood cyanide levels considered to be above the lethal threshold.1,8Following the administration of hydroxocobalamin, 10 of 14 subjects with cyanide poisoning (71%) survived.1
In this study, adverse events considered possibly to be caused by hydroxocobalamin were chromaturia (n=5), pink-to-red skin discoloration (n=3), increase in heart rate (n=1), and elevated blood pressure (n=1).8
How CYANOKIT Works
Cyanide Poisoning Prevents Cells From Using Oxygen
The ability of oxygen to access the cytochrome oxidase enzyme (present on the mitochondria inside cells) is essential to normal, life-sustaining cellular respiration. Cyanide poisoning may disable the body’s ability to use oxygen, so it can be fatal despite the amount of oxygen available to the body.1
Without treatment, exposure to a high dose of cyanide may result in death within minutes and may also cause central nervous system side effects, including altered mental status, parkinsonism, and personality changes.1,9
Studies show that the concentration of plasma lactate increases with the severity of cyanide poisoning and that hydroxocobalamin treatment results in rapid resolution of cyanide-induced lactic acidemia.2
Treat Known or Suspected Cyanide Poisoning With CYANOKIT
CYANOKIT is a cyanide antidote that contains hydroxocobalamin, a form of vitamin B12. Hydroxocobalamin binds to cyanide, creating nontoxic cyanocobalamin, allowing the body to use oxygen again.1
The Components of Each CYANOKIT
Each CYANOKIT consists of1: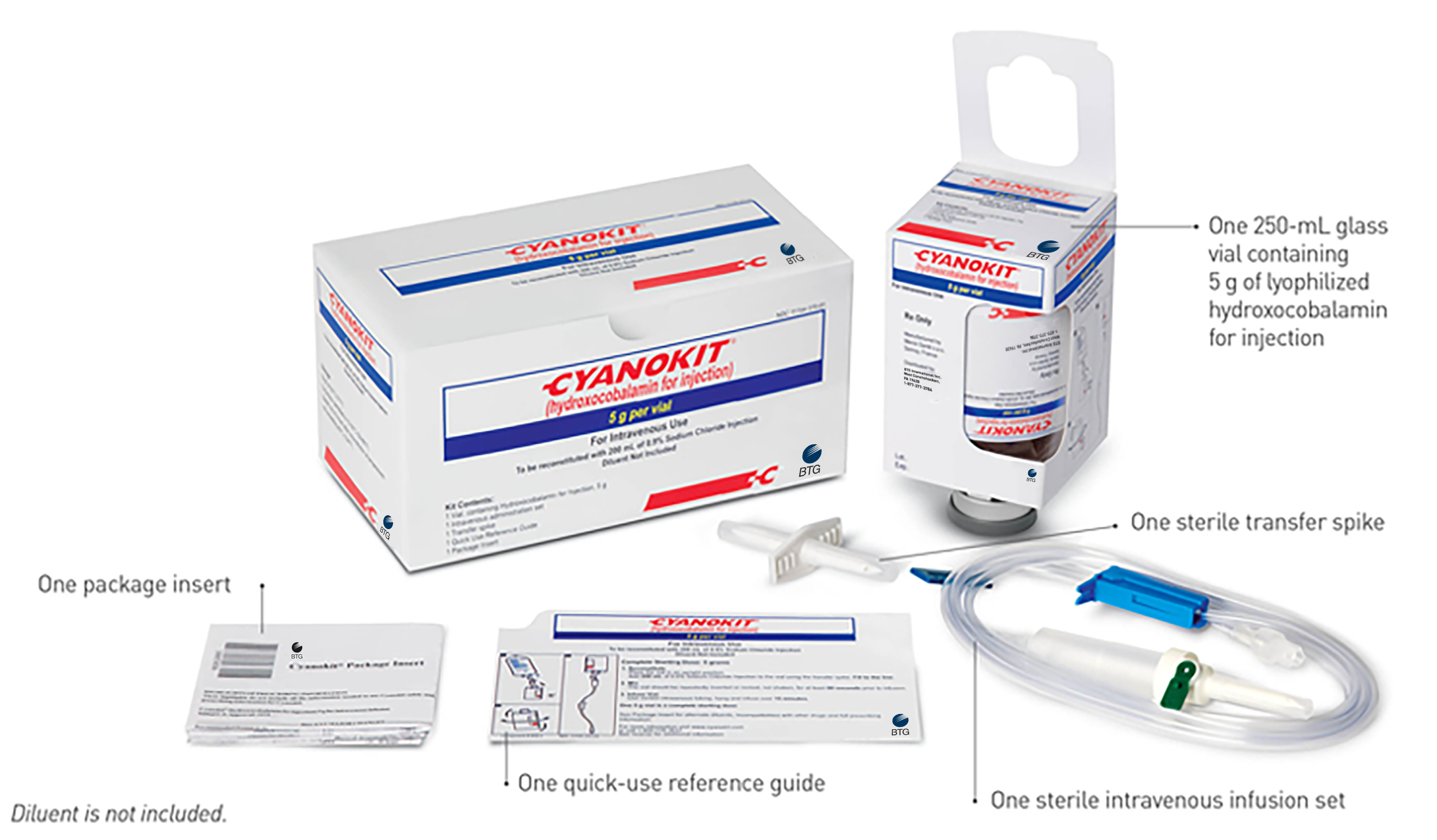
| NDC | Carton Dimensions |
|---|---|
NDC 50633-310-11 | W: 194 mm x L: 100 mm x H: 97 mm |
Dosing and Administration for CYANOKIT
Recommended Dosing for CYANOKIT1
- The starting dose of CYANOKIT for adults is 5 g (contained in a single vial), administered by IV infusion over 15 minutes (approximately 15 mL/min)*
- Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by IV infusion up to a total dose of 10 g
- The rate of infusion for a potential second dose may range from 15 minutes (for patients in extremis) to 2 hours, as clinically indicated
*No safety and efficacy studies have been performed in pediatric patients.
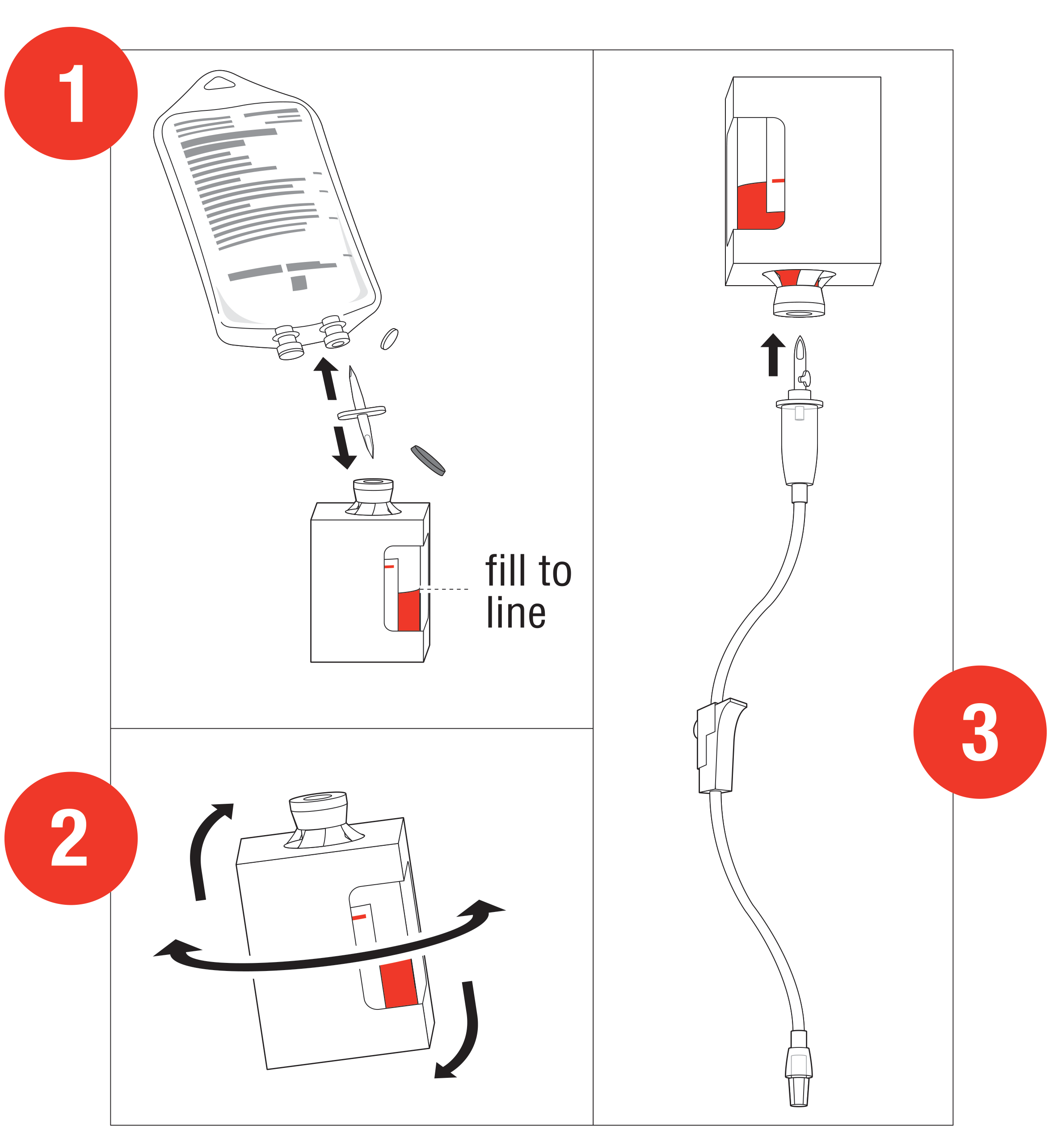
Preparation and Administration1
Starting dose: 5 g
- Reconstitute: Place the vial in an
upright position. Add 200 mL of 0.9% Sodium
Chloride injection† to the vial using the transfer
spike. Fill to the line.
†0.9% Sodium Chloride injection is the recommended diluent (diluent not included in the kit). Lactated Ringers injection and 5% Dextrose injection have also been found to be compatible with hydroxocobalamin and may be used if 0.9% Sodium Chloride is not readily available. - Mix: The
vial should be repeatedly inverted or rocked, not shaken, for at
least 60 seconds prior to infusion.
• CYANOKIT solutions should be visually inspected for particulate matter and color prior to administration
• Discard solution if particulate matter is present or solution is not dark red Infuse Vial: Use vented intravenous tubing, hang and infuse over 15 minutes.
The safety of administering other cyanide antidotes simultaneously with CYANOKIT has not been established. If a decision is made to administer another cyanide antidote with CYANOKIT, these drugs should not be administered concurrently in the same intravenous line.
CYANOKIT Incompatibility Information1
Physical incompatibility (particle formation) and chemical incompatibility were observed with the mixture of hydroxocobalamin in solution with select drugs that are frequently used in resuscitation efforts. Hydroxocobalamin is also chemically incompatible with sodium thiosulfate and sodium nitrite and has been reported to be incompatible with ascorbic acid. Therefore, these and other drugs should not be administered simultaneously through the same intravenous line as hydroxocobalamin.
Simultaneous administration of hydroxocobalamin and blood products (whole blood, packed red cells, platelet concentrate and/or fresh frozen plasma) through the same intravenous line is not recommended. However, blood products and hydroxocobalamin can be administered simultaneously using separate intravenous lines (preferably on contralateral extremities, if peripheral lines are being used).
Storing CYANOKIT1
To Use CYANOKIT Properly, Follow These Important Storage and Handling Guidelines
Lyophilized form: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). CYANOKIT may be exposed, during short periods, to temperature variations of usual transport, transport in the desert, and freezing/defrosting cycles.| Storage and transportation cycles | # of days | Temperature range |
|---|---|---|
| Usual transport | 15 | 5oC to 40oC (41oF to 104oF) |
| Desert transport | 4 | 5oC to 60oC (41oF to 140oF) |
| Freezing/defrosting cycles | 15 | -20oC to 40oC (-4oF to 104oF) |
Please see full Prescribing Information for complete instructions regarding dosing, preparation, administration, incompatibility, and storage information.